Why a Planned Baby Vaccine Trial in Guinea-Bissau Sparked a Global Ethics Debate
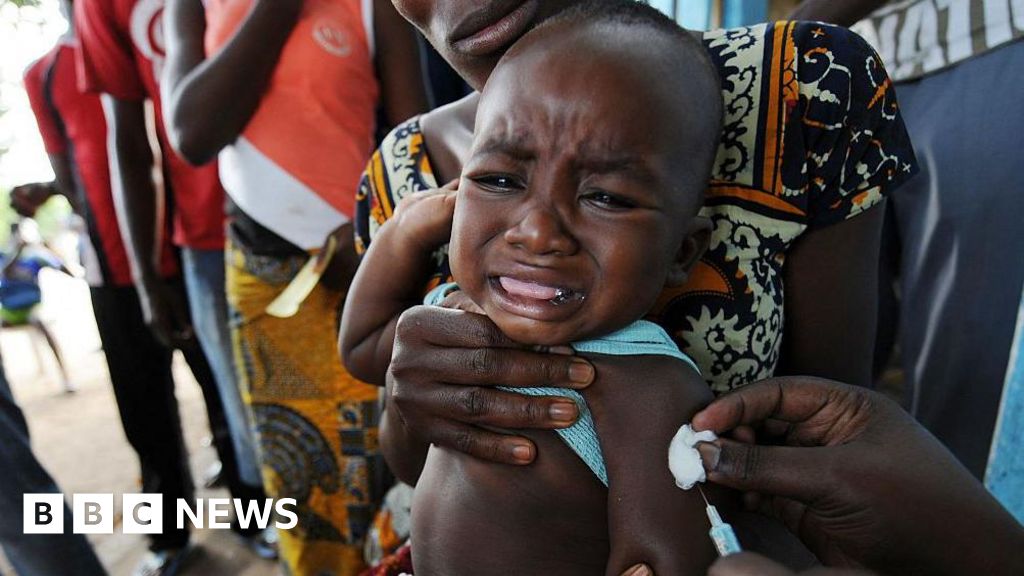
A planned US-funded baby vaccine trial in Guinea-Bissau has ignited a heated ethics debate. At the center is a simple but powerful question: when a vaccine is already recommended worldwide for newborns, is it acceptable to give it to some babies in a study—and withhold it from others?
The World Health Organization (WHO) has publicly criticized the planned trial, which would have given some infants an established hepatitis B birth dose while others followed the country’s delayed schedule. For parents, health workers, and anyone who cares about vaccine ethics, this story is unsettling but important: it shows how decisions about “standard of care” can literally become a matter of life and death.
What this article will help you understand
- What the Guinea-Bissau hepatitis B vaccine trial was planning to do
- Why the WHO and ethicists called the design “unethical”
- How global standards for the hepatitis B birth dose are set
- What this controversy means for parents, researchers, and policy makers
The backdrop: Hepatitis B and the newborn birth dose
Hepatitis B is a viral infection that attacks the liver. When babies are infected at birth or in early infancy, they are far more likely than adults to develop chronic infection, cirrhosis, or liver cancer later in life. This is why many countries have made the hepatitis B birth dose—a vaccine given within 24 hours of birth—a core part of their immunization programs.
The WHO has recommended this timely birth dose for years, particularly in countries with moderate to high hepatitis B prevalence. In many places, infants then receive additional doses in the first months of life as part of routine childhood vaccination schedules.

In Guinea-Bissau, however, the hepatitis B shot has historically been given later—starting at around six weeks of age. National authorities have already committed to introducing the birth dose nationwide by 2028 to align with global standards, with technical support from the WHO.
“Timely hepatitis B birth dose vaccination is a proven intervention to prevent chronic infection and its long-term consequences. Once a country commits to this standard of care, research must not roll it back.”
— Adapted from WHO guidance and expert commentary on hepatitis B vaccination
What was the planned US-funded trial in Guinea-Bissau?
According to reporting from the BBC and other outlets as of early 2026, the proposed US-funded study in Guinea-Bissau aimed to examine the impact of introducing the hepatitis B birth dose in this specific context. While the full protocol has not been made publicly available in detail, key reported features included:
- Newborns in the study would be divided into at least two groups.
- One group would receive the hepatitis B birth dose shortly after delivery, consistent with WHO global recommendations.
- Another group would follow the existing national schedule, receiving their first hepatitis B shot at around six weeks of age.
- Researchers would then compare outcomes between the groups—potentially including infection markers, immune responses, and other health indicators.
On paper, this might look like a typical “implementation science” trial: testing how a globally recommended intervention performs in a specific low-resource setting. But the controversy centers on a crucial ethical question: once a birth dose is accepted as the global standard of care, can you justify withholding it from some babies in the name of research?
Why the WHO and others called the trial “unethical”
The WHO’s criticism focused on the basic design of giving some babies a recommended vaccine at birth while others did not receive it until weeks later. In their view, this created an avoidable gap in protection for infants randomized to the delayed schedule.
Key ethical concerns
- Standard of care: Once a global health standard exists and a country has committed to it, deliberately keeping a subset of babies on an older, less-protective schedule raises red flags.
- Equipoise: Clinical trials are supposed to operate under “genuine uncertainty” about which option is better. In this case, strong global evidence already favors early hepatitis B vaccination.
- Vulnerability: Newborns in low-income settings are among the most vulnerable research participants. Ethics bodies generally insist on extra safeguards, not weaker ones.
- Justice: There are longstanding concerns about using populations in lower-income countries to test interventions that wealthier countries already consider standard care.
“If we would not run this kind of trial in a high-income country, we should think very hard before running it in a low-income one—especially when it involves withholding a recommended vaccine from newborns.”
— Paraphrased view shared by several global health ethicists commenting on the case
WHO officials have indicated they would work directly with Guinea-Bissau’s health authorities to support nationwide rollout of the birth dose by 2028 without exposing part of the newborn population to delayed protection for research purposes.
What the science says about hepatitis B birth dose vaccines
The controversy in Guinea-Bissau doesn’t arise in a vacuum; it’s shaped by decades of research on hepatitis B vaccination. Large observational studies and program evaluations from Asia, Africa, and the Pacific Islands have consistently shown:
- Earlier is better: Giving the first dose within 24 hours of birth significantly lowers the risk of chronic hepatitis B infection compared with waiting until several weeks of age.
- Added benefit for babies born to infected mothers: In settings where maternal screening is limited, a universal birth dose helps protect infants whose mothers have undiagnosed hepatitis B.
- Long-term impact: Countries that adopted birth dose plus follow-up doses have seen major declines in hepatitis-B–related liver cancer in younger adults.
These findings underpin current WHO recommendations and the push for universal birth dose coverage, particularly in low- and middle-income countries.
Guinea-Bissau’s reality: Why timing is such a challenge
To understand why a delayed schedule has persisted in Guinea-Bissau, it helps to look at day-to-day realities on the ground. In many low-income settings:
- Many births happen at home or in facilities without reliable cold-chain storage.
- Families may need to travel long distances to reach clinics.
- Health systems are stretched thin, with limited staff and resources.
A nurse in a rural West African clinic once described to a global health researcher (in a case study shared at a vaccine policy workshop) how she carried vaccines in a cooler on the back of a motorbike to reach home births before the 24-hour window closed. Sometimes she made it in time; often, she did not. Her story echoes the broader struggle: the science is clear, but implementation is hard.

These challenges are real—and they make it crucial to study how best to deliver vaccines. But, as the WHO and many ethicists argue, such studies should look for ways to bring more babies up to the global standard, not deliberately keep some on a slower path.
Bigger lessons: Ethics, equity, and trust in global vaccine research
The fallout from the Guinea-Bissau trial debate extends far beyond a single study. It touches on long-running concerns about how global health research is designed and who benefits.
1. Research must not downgrade care for the sake of data
When effective interventions already exist, ethical trial designs typically compare:
- Standard of care vs. standard of care plus an enhancement (e.g., extra outreach, new delivery strategies), not “standard vs. substandard.”
- Different implementation strategies that all meet or exceed minimal acceptable care.
2. Communities deserve a real voice
Parents and local leaders in countries like Guinea-Bissau are not passive subjects. Increasingly, ethics guidance emphasizes:
- Early community engagement in choosing research questions.
- Transparent explanation of risks, benefits, and alternatives.
- Long-term investment in local health systems, not just short-term studies.
3. Trust is fragile—and essential
If people see that babies in their communities are being given less than the global standard in the name of research, trust in vaccines and health authorities can erode quickly. That mistrust can spill over into resistance to other lifesaving interventions, from measles shots to maternal care.
Practical takeaways for parents, advocates, and health workers
While the Guinea-Bissau situation is specific, it offers some practical lessons you can carry into conversations about vaccines and ethics wherever you live.
For parents and caregivers
- Ask your health provider which vaccines are recommended at birth and why.
- If you’re in a region where hepatitis B is common, discuss the timing of the first dose and any barriers to getting it promptly.
- When hearing about “vaccine trials,” feel free to ask how participants are protected and what the standard of care is.
For health advocates and journalists
- Frame discussions around both benefits (disease prevention) and rights (fair access to standard care).
- Highlight examples where ethical research has strengthened, not weakened, health systems.
- Use neutral, evidence-based language; avoid overstating risks or benefits.
For clinicians and local health workers
- Stay updated on WHO and national guidelines for hepatitis B and other newborn vaccines.
- Advocate for better logistics—cold-chain support, staff training, home visit systems—to make birth dose delivery feasible.
- If approached about participating in a trial, review the study’s ethical safeguards and consult your local ethics committee.

Common questions about the Guinea-Bissau vaccine trial story
Was the hepatitis B vaccine itself experimental?
No. The hepatitis B vaccine used in birth dose programs worldwide is an established vaccine with a long track record of safety and effectiveness. The issue in Guinea-Bissau was not about testing a new, unproven vaccine but about comparing different timings of an existing one.
Does the WHO oppose vaccine research in low-income countries?
No. The WHO actively supports research in all settings, including low-income countries. Their concern in this case was that the proposed design appeared to conflict with ethical standards by potentially depriving some newborns of timely access to a recommended vaccine.
Should parents be worried about their babies getting the hepatitis B birth dose?
For most families, the hepatitis B birth dose is a routine, well-studied part of infant care. Public health authorities in many countries endorse it because the benefits—reduced risk of chronic infection and liver disease—are well established. If you have specific concerns, it is always appropriate to discuss them with your healthcare provider.
Moving forward: Protecting babies and protecting ethics
The uproar over the US-funded hepatitis B vaccine trial in Guinea-Bissau is uncomfortable, but it may ultimately be healthy. It has forced funders, regulators, and researchers to look closely at how we balance the desire for data with the duty to protect the most vulnerable—newborns in low-resource settings.
As Guinea-Bissau prepares to roll out the birth dose nationwide by 2028 with WHO support, the real challenge is not whether infants deserve timely protection—they do—but how to make that promise real in every village, clinic, and delivery room.
Wherever you stand in this story—as a parent, health worker, student, or concerned citizen—you have a role. You can ask hard questions about research ethics, support evidence-based vaccination, and amplify the message that no child’s standard of care should be lowered for the sake of a study.
Article metadata (SEO & schema)
Below is structured data in JSON-LD format to help search engines understand this page as a news-style article about vaccine ethics and hepatitis B birth dose policy.
